| PARAMETER | U.S.P. | Result |
| Appearance | White powder | White powder |
| pH | 5.5 -7.0 | 6.4 |
| Moisture | 7%max | 6.7% |
| Loss on drying | 5%max | 4.1% |
| Water Insoluble Substances | 0.25max | 0.18% |
| Residue on ignition | 0.5%max | 0.2% |
| Heavy metals | 10ppm max | 40ppm |
| Arsenic | 2ppm max | 1.6ppm |
| Assay | 97%- 102% | 97.5% |
| Conductivity | NMT-75 µs/cm | 50µs/cm |
| Starch | Negative | Negative |
| aerobic bacteria
|
1000CFU/G max | 90CFU/G |
| molds and yeast
|
100CFU/G max | 90CFU/G |
| Escherichia coli
|
Non detected | Non detected |
Microcrystalline cellulose
Prepared at the 49th JECFA (1997)
superseding specifications prepared at the 46th JECFA (1996),
published in FNP 52 Addendum 4 (1996)
| SYNONYMS | Cellulose gel, INS No. 460 |
| DEFINITION | Microcrystalline cellulose is a purified, partially depolymerized cellulose prepared by treating alpha-cellulose, obtained as a pulp from fibrous plant material, with mineral acids. The degree of polymerization is typically less than 400. Not more than 10% of the material has a particle size of less than 5聽m聽m. |
| Chemical names | Cellulose |
| C.A.S. number | 9004-34-6 |
| Chemical formula | (C6H10O5)n |
| Assay | Not less than 97% of carbohydrate calculated as cellulose on the dried basis |
| DESCRIPTION | Fine, white or almost white, odourless, free flowing crystalline powder |
| FUNCTIONAL USES | Emulsifier, stabilizer, anticaking and dispersing agent |
| CHARACTERISTICS | |
| IDENTIFICATION | |
| Solubility | Insoluble in water, ethanol, ether and dilute mineral acids. Slightly soluble in sodium hydroxide solution |
| Infrared absorption | The infrared absorption spectrum of a potassium bromide dispersion of the sample corresponds to the infrared spectrum in the Appendix |
| PURITY | |
| Loss on drying | Not more than 7.0% (105 掳, 3h) |
| pH | 5.0 – 7.5 Shake 5 g of the sample with 40 ml of water for 20 min and centrifuge. Use the supernatant for pH determination |
| Water soluble substances | |
| Not more than 0.24% See description under TESTS |
|
| Sulfated ash | Not more than 0.05% Proceed as directed under the test for Ash (Sulfated Ash, Method I) using 2 g of the sample |
| Lead | Not more than 2 mg/kg Prepare a sample solution as directed for organic compounds in the Limit Test and determine the lead content by atomic absorption spectrometry |
| Starch | Not detectable See description under TESTS |
| TESTS | |
| PURITY TESTS | |
| Water soluble substances | |
| Shake 5 g of the sample with approximately 80 ml of water for 10 min, filter through Whatman No. 42 or equivalent filter paper into a tared beaker, evaporate to dryness on a steam bath and dry at 105掳 for 1h. Cool, weigh and calculate as percentage. | |
| Starch | Mix 30 g of the sample with 270 ml of water in a high-speed (18,000 rpm) blender for 5 min. Transfer 100 ml of the mixture to a 100-ml graduated cylinder, and allow to stand for 3h. A white opaque, bubble-free dispersion which does form a supernatant liquid at the surface, is obtained.
To 20 ml of this dispersion add a few drops of iodine TS, and mix. No purplish to blue or blue colour should be produced. |
| METHOD OF ASSAY | Transfer about 125 mg of the sample, accurately weighed, to a 300-ml Erlenmeyer flask, using about 25 ml of water. Add 50.0 ml of 0.5N potassium dichromate, mix, then carefully add 100 ml of sulfuric acid, and heat to boiling. Remove from heat, allow to stand at room temperature for 15 min, cool in a water bath, and transfer into a 250-ml volumetric flask. Dilute with water almost to volume, cool to 25 掳, then dilute to volume with water, and mix. Titrate a 50.0-ml aliquot with 0.1N ferrous ammonium sulfate, using 2 or 3 drops of ortho-phenanthroline TS as the indicator, and record the volume required as S, in ml. Perform a blank determination, and record the volume of 0. IN ferrous ammonium sulfate required as B, in ml. Calculate the percentage of cellulose in the sample by the formula:
in which W is the weight of sample taken, in mg, corrected for Loss on Drying. |
MCC as Wet Granulation Filler : MCC is one of the very few types of filler with water insoluble yet hydrophilic properties with swelling tendencies (other examples being calcium pectinate and sodium alginate) and excellent water imbibing or wicking action. This is what makes it an excipient of choice in wet granulation procedures. Both Avicel PH 101 and PH 102 can be used advantageously as fillers in wet granulation in a recommended level of 5-15%. When used as a wet massing adjunct, the wicking action of MCC promotes rapid and even wetting of the powder mix. An advantage of its use in wet granulation is its ability to retain water, which makes the wet mass less sensitive to overwetting due to an excess of granulating fluid. The milling of the wet mass is easier due to less clogging of the screen that produces a more uniform granulation, which is readily dried and reduces case hardening. Case hardening is a phenomenon observed in incompletely dried granules. In some cases, when the granules are dried at a high temperature, the inside of the granules remain wet and the surface seems dried. The granules are often hard and resist disintegration. Under compaction forces, the granules break and deform plastically to form soft tablets due to the moisture coming out of the incompletely dried granules.
The addition of Avicel PH 101 or PH 302 in wet granulation promotes rapid, even wetting as a result of the wicking action of MCC, reduced sensitivity of the wet mass to over-wetting, faster drying, fewer screen blockages or case hardenings, reduced dye migration, and faster disintegration. [6]Since MCC is water insoluble, the small drug particles get trapped between the deformed MCC particles and may delay wetting and dissolution. This must be overcome by adding portions of water soluble direct compression excipients. [4]Roller Compaction : Roller compaction is a dry process involving compaction of materials into ribbons that are then milled to generate a granulation. This granulation is then lubricated and compressed on a tablet machine. This process can be used with moisture-sensitive active pharmaceutical ingredients and is readily adaptable to continuous processing. Use of Avicel PH grades in roller compaction includes improvement of compaction in the ribbon phase, enhancement of flow of the granules, and preserving content uniformity of the final granulation.
MCC as Directly Compressible Filler: MCC is the most compressible of all the direct compression fillers and has the highest dilution potential and capacity, which is defined as the amount of active ingredient that a diluent can successfully carry in the direct compression technique. [4] This can be explained on the basis of the nature of MCC particles themselves, which are held together by hydrogen bonds in the same way that a paper sheet or an ice cube is bonded. Hydrogen bonds on adjacent cellulose molecules account solely for strength and cohesiveness. MCC particles are deformed plastically under compaction forces to yield an extremely large number of clean surfaces brought in contact during this deformation forming a strong compact even under low compression forces. [5]
Another factor of MCC being the most favorite diluent is its low bulk density. An excipient with low bulk density and large particle size distribution will exhibit a high dilution potential on a weight basis, optimum packing density, and coverage of drug and other excipient materials. [4]Avicel grades (Avicel PH-102 SCG, Avicel HFE-102, Avicel PH-200, Avicel PH-302) provide excipient solutions to many challenges of direct compression formulations including improved flow, better compressibility, and accommodation of moisture-sensitive actives. [6]Overall, as direct compression filler, Avicel promotes efficient dry blending of ingredients and produces tablets with high hardness levels and low friability levels with excellent compression. It produces tablets of superior whiteness and color stability.
Microcrystalline cellulose (MCC) has emerged as the most resourceful excipient of all times in drug research. Thanks to its profusion in terms of grades available for different needs and its physical properties that support a variety of functionality requirements especially for the most frequently used unit dosage forms. MCC can be used as a bulking agent, disintegrant, binder, lubricant, and glidant besides being a stability enhancer and a secondary suspending agent. It can be used in direct compression of most drugs and saves material, capital, equipment, and labor. Its ever increasing applications in drug research include its utility in immediate release (tablets and liquids) dosage forms, sustained release dosage forms (multiparticulates and matrix tablets), topical preparations, oral liquids, organoleptic enhancements as in chewable and mouth dissolving tablets, anti-reflux, and nutraceuticals. The review discusses these applications in sufficient detail citing examples and investigating the justifications for such functions.
Despite many research studies being conducted in the field of drug delivery, none of them have been able to match the characteristic features and advantages offered by oral unit dosage forms such as tablets and capsules. With the advent of this form of drug delivery, the subsequent researches of betterment of this delivery system emerged, which included sophisticated machinery and superior tabletting aids. These tabletting aids, which we affectionately call excipients, have been an imperative part of tabletting that forms the basis of delivering potent or non potent drugs in the form of such effective and patient-friendly unit dosage forms. The most basic and important excipients categories involved in tabletting include diluents/bulking agents, disintegrants, binders, lubricants, and glidants and many examples of each can be cited from the oldest to the newest literature. Each of these categories of excipients has a specific proven cause for its requirement in tabletting and the absence of which can cause serious consequences. Many excipients offer multiple advantages and can serve dual purposes. However, microcrystalline cellulose (MCC) is an excipient that can play the roles of almost every category listed above for tablet research. Because of this, MCC is the most versatile and user friendly excipient among numerous available. The development of MCC has made an extremely valuable tabletting agent available to the pharmaceutical industry and since its introduction in late 1950s, it stands today as the single most important tablet excipient developed in modern times. Its incorporation in one way or the other can be seen in almost every finished unit dosage form.
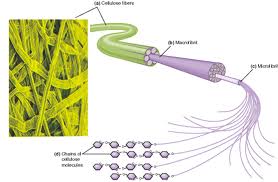
MCC is derived from a special grade of purified alpha wood cellulose by severe acid hydrolysis to remove the amorphous cellulose portions, yielding particles consisting of bundle-like needle-like microcrystals